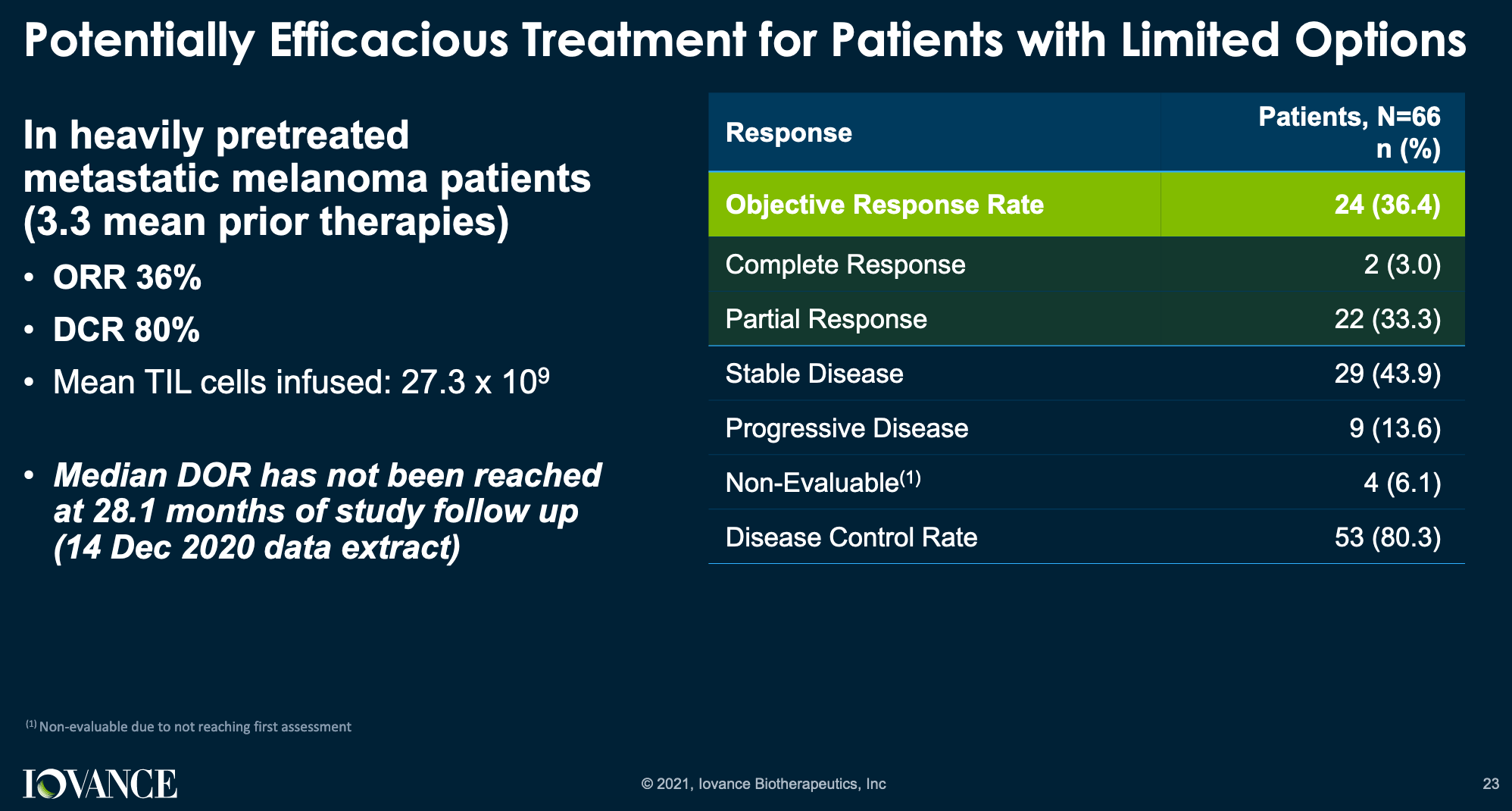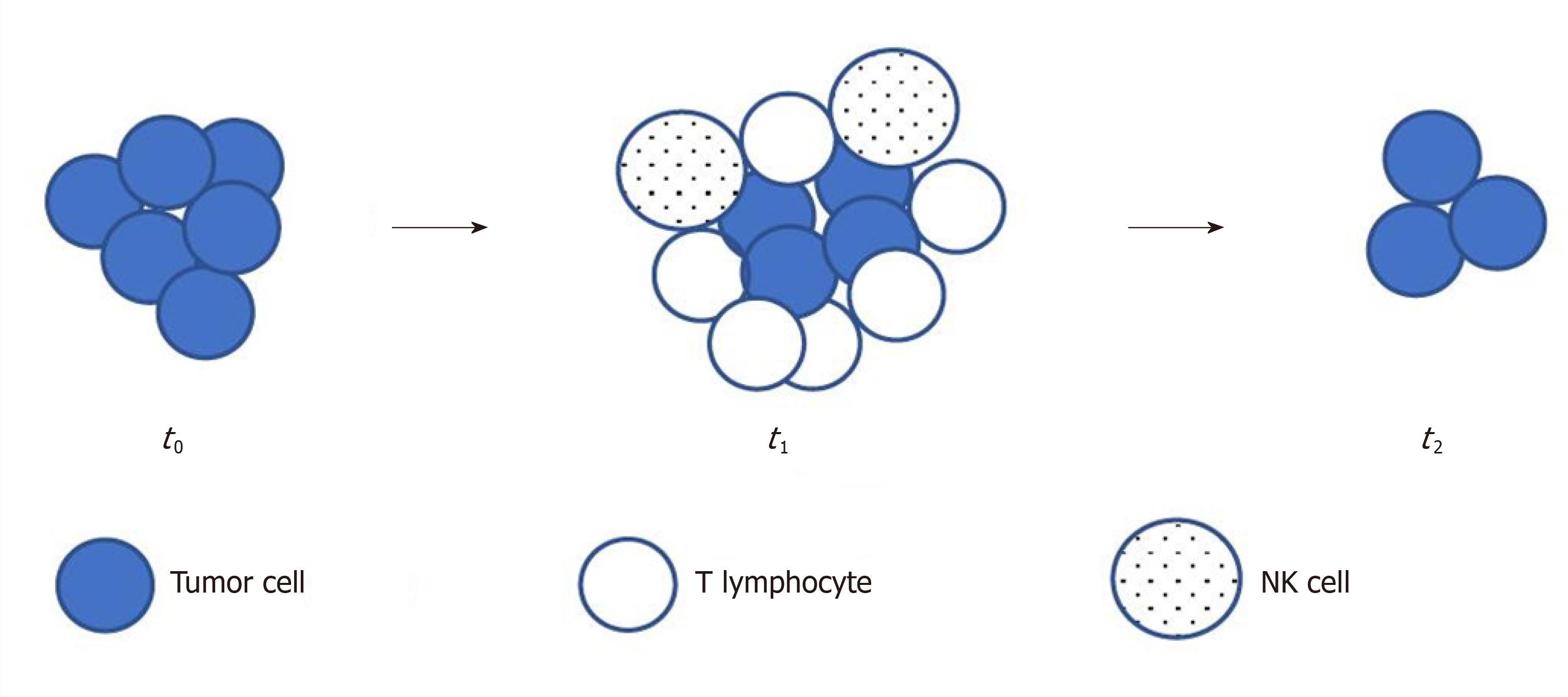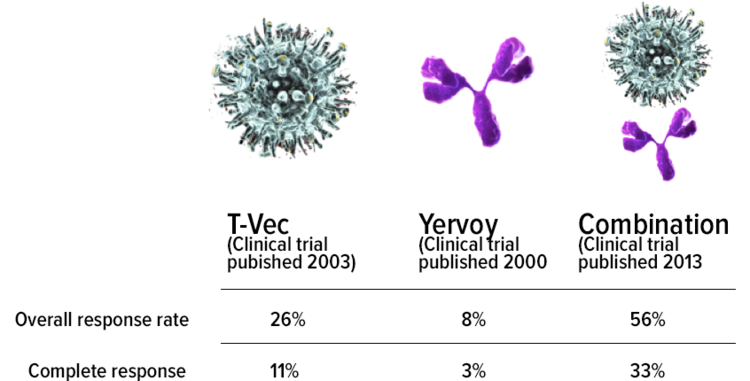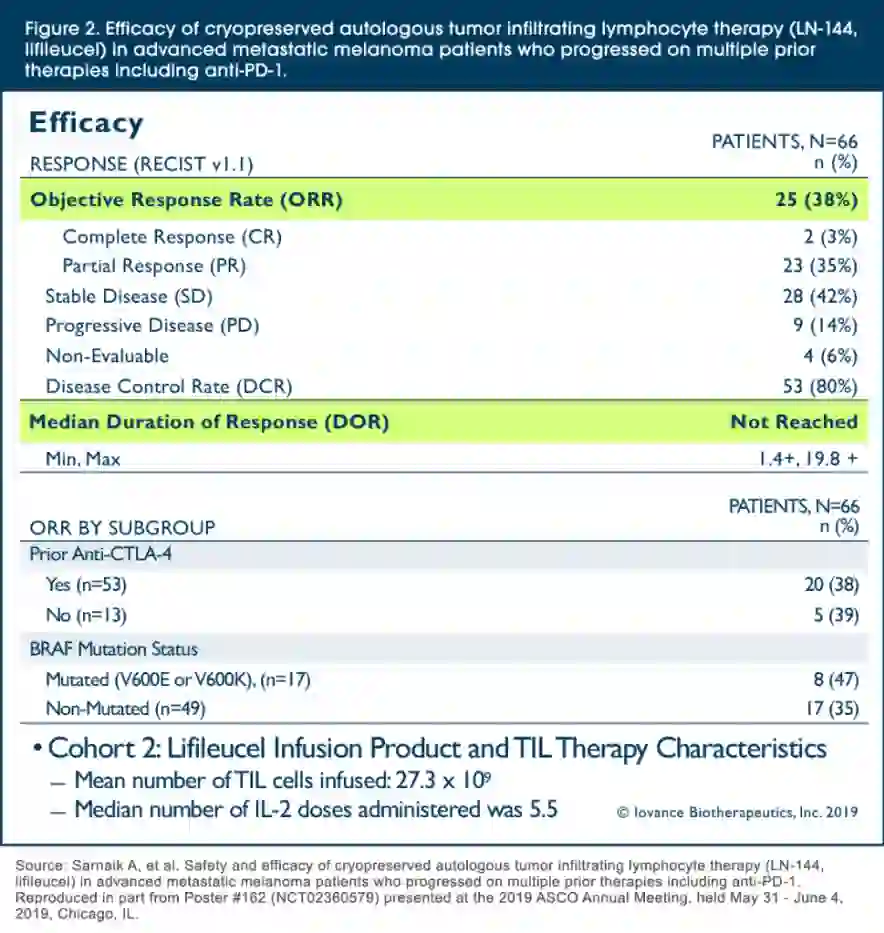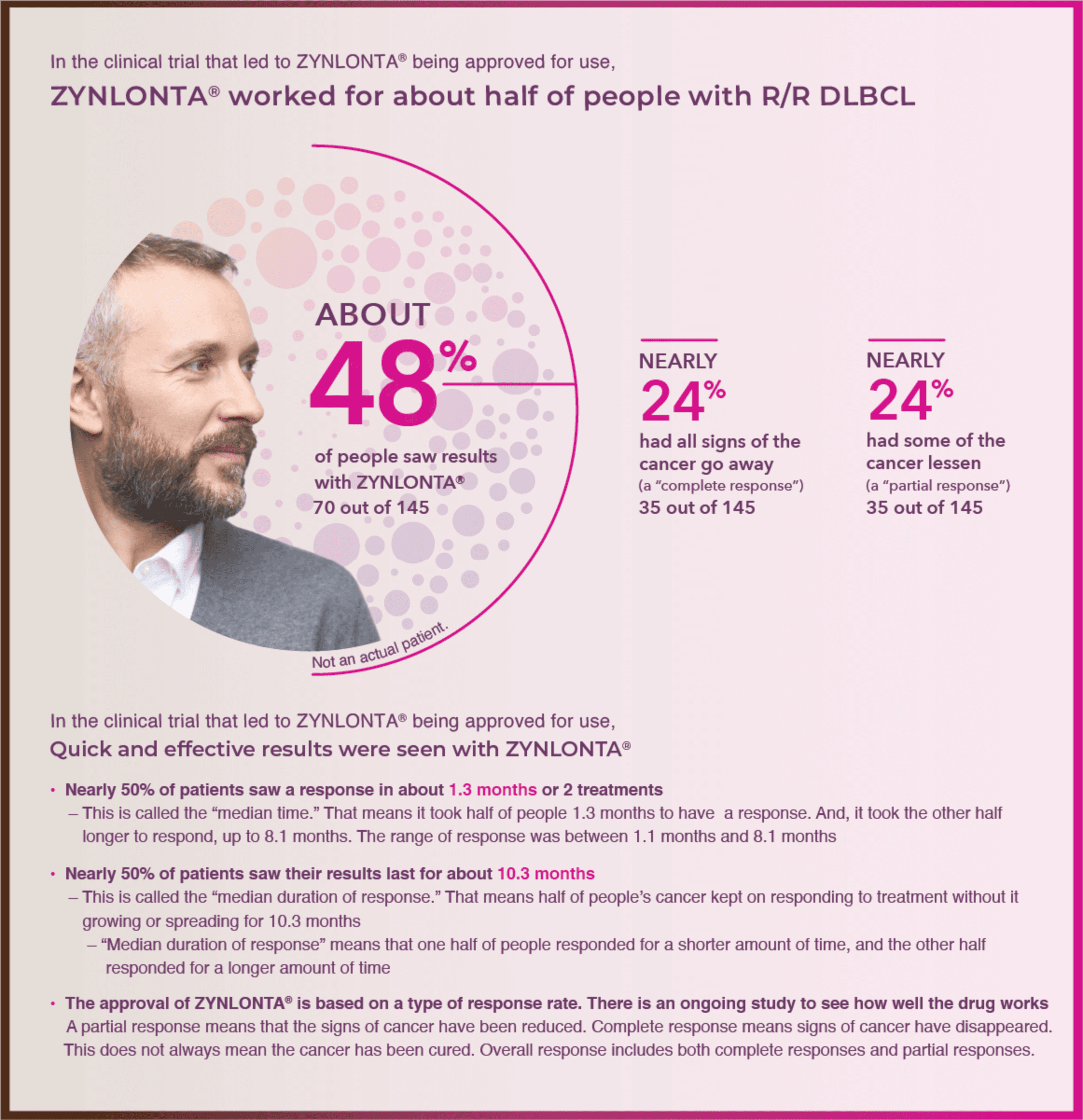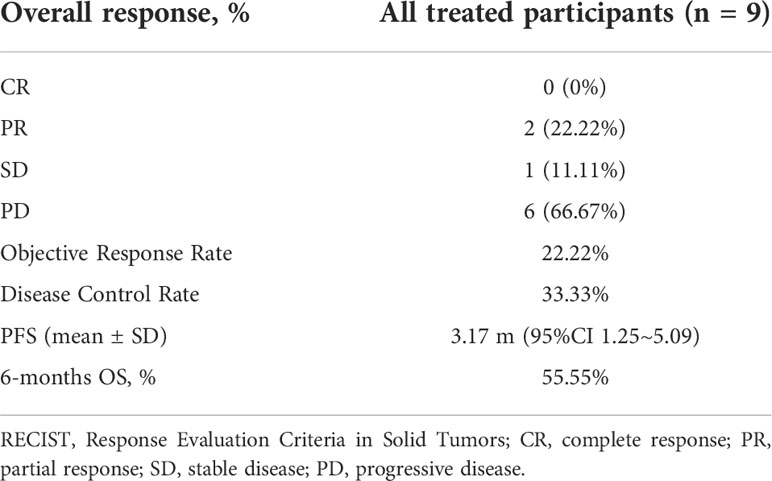
Frontiers | Investigation of the efficacy and safety of cryoablation and intra-arterial PD-1 inhibitor in patients with advanced disease not responding to checkpoint inhibitors: An exploratory study
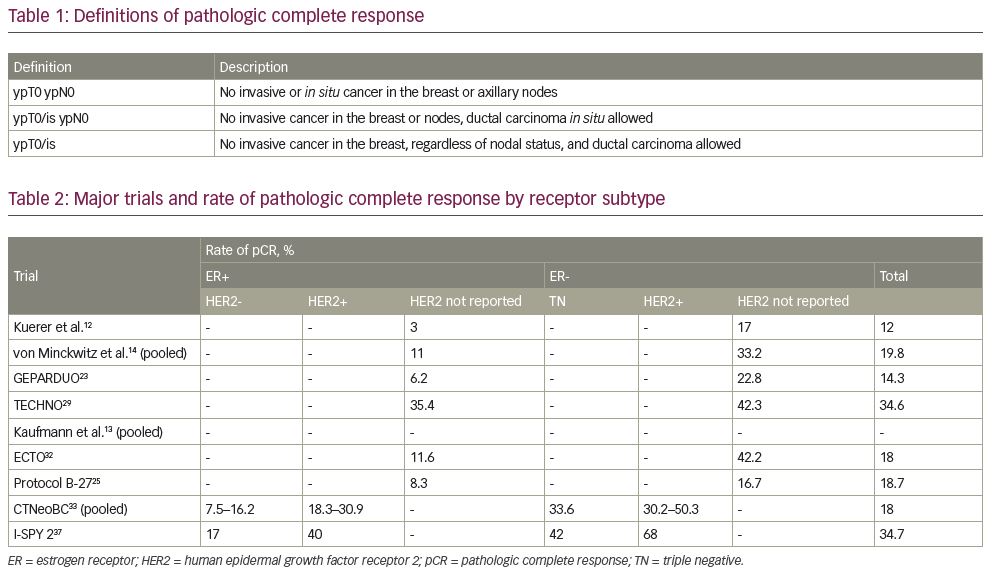
Physical exercise during neoadjuvant chemotherapy for breast cancer as a mean to increase pathological complete response rates: Trial protocol of the randomized Neo-ACT trial | PLOS ONE
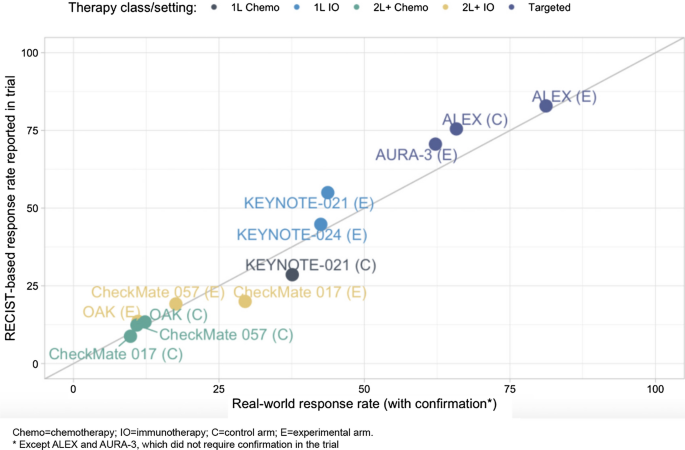
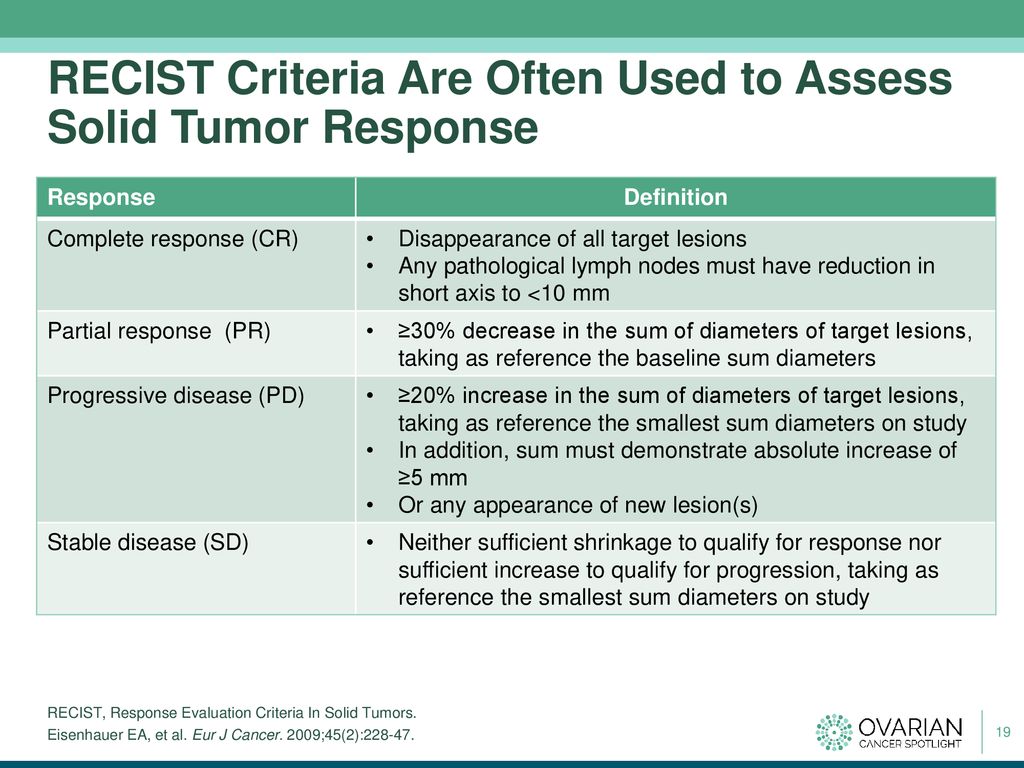
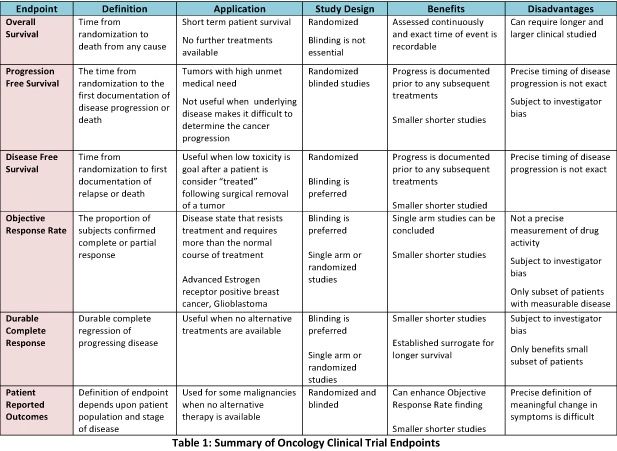
Characterization of a Real-World Response Variable and Comparison with RECIST-Based Response Rates from Clinical Trials in Advanced NSCLC | SpringerLink

Overall Response Rate, Progression-Free Survival, and Overall Survival With Targeted and Standard Therapies in Advanced Non–Small-Cell Lung Cancer: US Food and Drug Administration Trial-Level and Patient-Level Analyses | Journal of Clinical Oncology
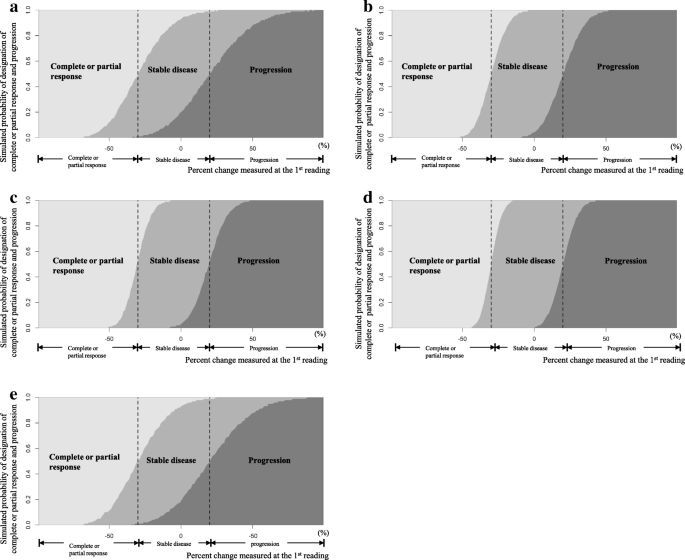
Development of an algorithm for evaluating the impact of measurement variability on response categorization in oncology trials | BMC Medical Research Methodology | Full Text
Physical exercise during neoadjuvant chemotherapy for breast cancer as a mean to increase pathological complete response rates: Trial protocol of the randomized Neo-ACT trial | PLOS ONE
KAZIA PRESENTS FURTHER PAXALISIB AND CANTRIXIL DATA AT AACR, REINFORCING POSITIVE EFFICACY SIGNALS FOR BOTH DRUGS