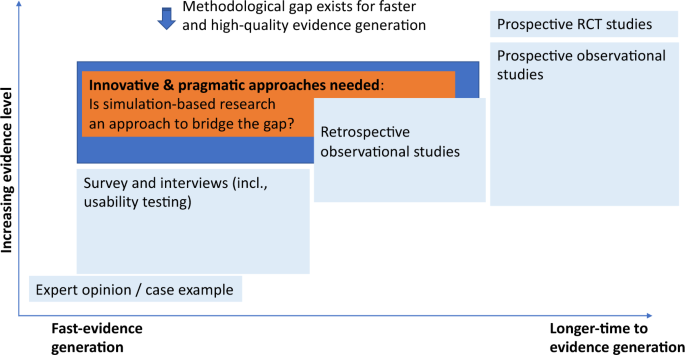
Challenges for the evaluation of digital health solutions—A call for innovative evidence generation approaches | npj Digital Medicine
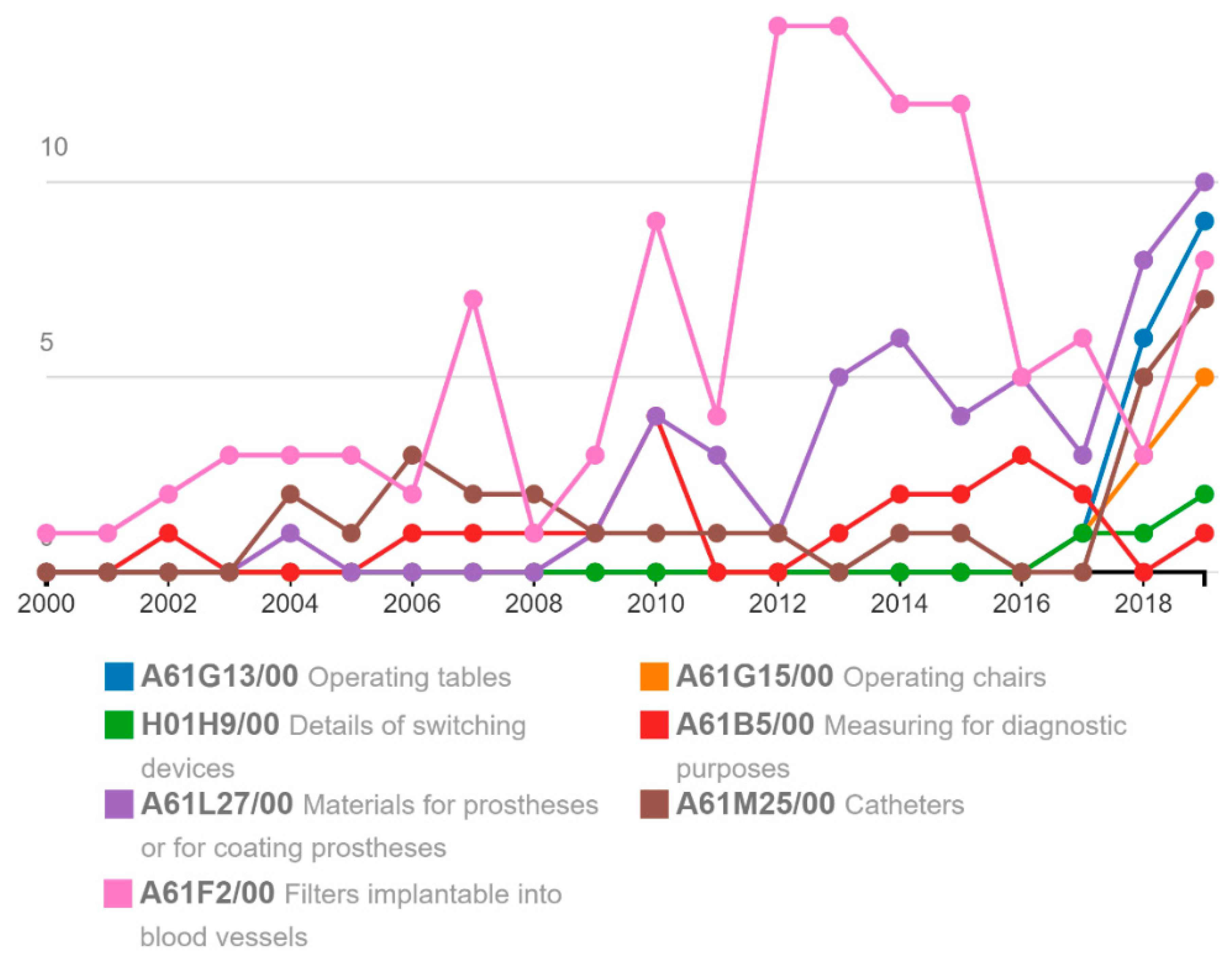
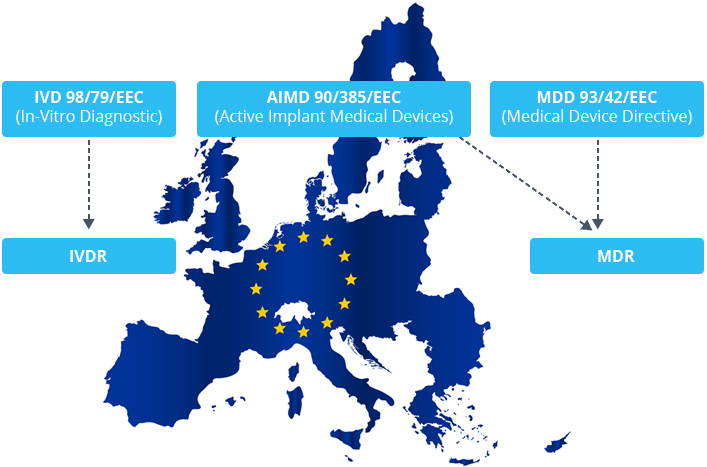
Administrative Sciences | Free Full-Text | New Regulations on Medical Devices in Europe: Are They an Opportunity for Growth? | HTML

Conducting Non-COVID-19 Clinical Trials during the Pandemic: Can Today's Learning Impact Framework Efficiency? in: European Journal of Health Law Volume 27 Issue 5 (2020)
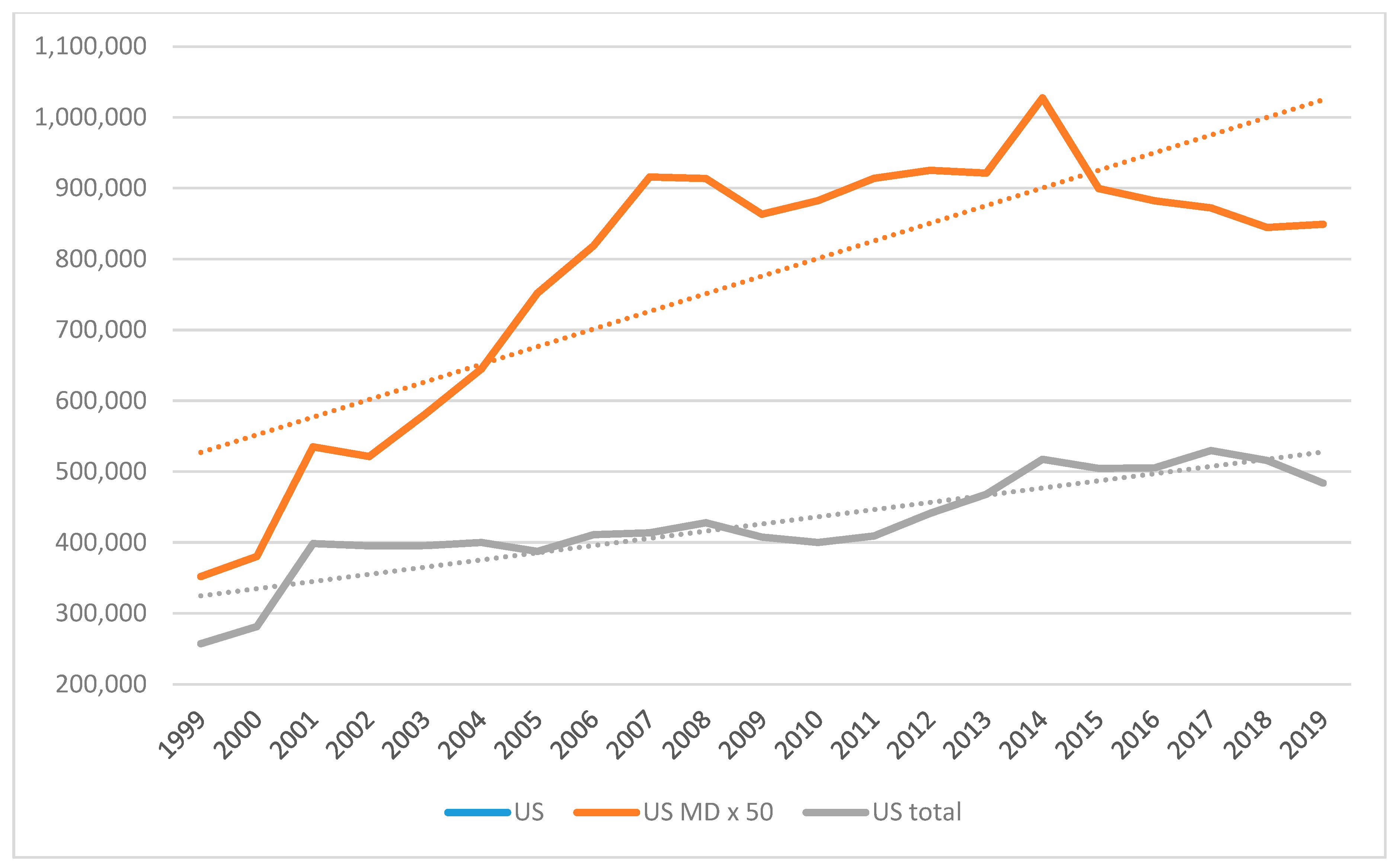
Administrative Sciences | Free Full-Text | New Regulations on Medical Devices in Europe: Are They an Opportunity for Growth? | HTML

Mapping the teaching of honeybee veterinary medicine in the European Union and European Free Trade Area. - Abstract - Europe PMC

Conducting Non-COVID-19 Clinical Trials during the Pandemic: Can Today's Learning Impact Framework Efficiency? in: European Journal of Health Law Volume 27 Issue 5 (2020)